Media
HKU Scientists Effectively Suppress Tumour Growth
by Converting Salmonella into YB1 Anaerobe Bacterium
25 Apr 2016
(From Left) Dr Bin Yu, Post-doctoral fellow, Professor Jiandong Huang, Professor, and Dr Lei Shi, Research Assistant of School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, HKU successfully converted Salmonella into an anaerobe bacterium “YB1” which can suppress tumour growth.
Salmonella is a causative agent of food poisoning and can be commonly found in inadequately cooked meat, raw egg and egg products. Common symptoms include vomiting, diarrhoea and abdominal pain. Researchers at School of Biomedical Sciences of Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU) have recently engineered the Salmonella into an anaerobe bacterium named YB1 which can only survive and thrive in the hypoxic condition, for example, hypoxic regions inside solid tumours. YB1 can effectively inhibit the growth of tumours while being safe to normal tissues. This invention has been filed patent applications in different countries through the Technology Transfer Office of HKU. A patent was recently granted by the United States Patent and Trademark Office. The researchers hope that YB1 can be further developed into a tumour-targeting agent in the near future.
The “obligate” anaerobe YB1
Using an advanced DNA engineering method of recombineering and the concept of synthetic biology, research team at the School of Biomedical Sciences of Li Ka Shing Faculty of Medicine, HKU successfully converted the bacteria, Salmonella typhimurium, into an “obligate” anaerobe, namely YB1. The virulence of Salmonella is preserved in YB1, but having an add-on characteristic that it can only survive in anaerobic conditions (such as hypoxic regions inside solid tumours), thus it does minimal damage to the aerobic normal tissues. Therefore, YB1 is safe to normal tissues, but selective to tumour where hypoxia exists. This property makes it much safer as a great clinical tool to fight against cancer in the near future.
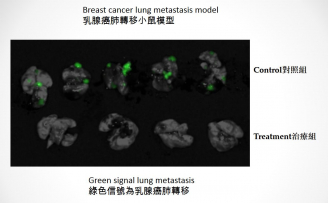
YB1 has been tested in liver and breast cancer mouse models for its efficacy and safety as a cancer treatment vector. Results showed that YB1 was able to colonise inside the tumour and effectively suppress cancer metastasis. In contrast, YB1 is quickly eliminated in normal tissues as designed. In a breast cancer mouse model, tumour growth was reduced by 50% and cancer metastasis was completely inhibited when treated with YB1. In a liver cancer mouse model, tumour growth was remarkably suppressed by 90% in the YB1 treated mice compared to the non-treated mice. Furthermore, YB1 was able to inhibit the growth of other solid tumours, for example, neuroblastoma.
Future Research
The research team is currently exploring other potential use of this new bacterium. The lead scientist of this research, Professor Jiandong Huang at the School of Biomedical Sciences of Li Ka Shing Faculty of Medicine said, “Owing to the property that YB1 can survive in tumour, it can be viewed as a ‘guided missile’ to deliver destructive ‘warheads’ to the tumour tissue by means of delivering therapeutic proteins and drugs, resulting in tumour regression.” In order to further improve the efficacy, YB1 was equipped with different therapeutic drugs. In a breast cancer mouse model, YB1 carrying Diphtheria toxin A not only completely suppressed tumour growth but also reduced tumour size by inducing cancer cell death. In total, tumour completely regressed in 26% of mice treated with the bacterium. Mice treated with the bacterium were able to survive during the whole experiment which lasted for 4 weeks after treatment, whereas mice in the control group all died on around Day 15. It is estimated that YB1 will be ready for clinical trials in a few years.
Research Background
Cancer is one of the most deadly diseases. The current therapeutic methods such as chemotherapy or radiation therapy cannot cure a large portion of cancer patients. Due to the rapid proliferation of cancer cells and inefficient tumour vascular supply, hypoxia occurs in all solid tumours as a result of an inadequate supply of oxygen. Hypoxia in tumour is associated with resistance to therapy. Hypoxia induces cellular adaptations to the stressful environment, preventing cell death and promoting the progression of tumours, and therefore compromises the effectiveness of chemotherapy and radiotherapy.
In recent years, using bacteria as therapeutic agents against solid tumours becomes an emerging field in cancer therapy research. Infections with anaerobic bacteria have been known in some cases to cause partial or complete regression of malignant tumours since the 1890s. However, the wild-type bacteria can cause severe infection, resulting in death of patients. At that time, researchers did not know how to control the infection of bacteria and improve the treatment outcome. This situation begins to change with the development of DNA engineering technology and synthetic biology. Bacteria can now be genetically engineered for cancer therapy.
About the research team
The research was led by Professor Jiandong Huang, Professor of the School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, HKU. Other research team members include Post-doctoral Fellow Dr Bin Yu, Research Assistant Dr Lei Shi, Research Associate Dr Qiubin Lin, PhD student Miss Chen Xu of Li Ka Shing Faculty of Medicine, HKU, as well as Dr Miki Lee, Dr Mei Yang.
About Technology Transfer Office at HKU
The Technology Transfer Office (TTO) manages the use of the intellectual property assets of HKU by providing patenting, licensing, and other commercialisation support to our researchers. Acting as the bridge linking HKU to society in the arena of technology commercialisation, TTO facilitates industries and businesses to access HKU’s powerhouse of knowledge, innovation, and expertise through close collaboration. For HKU’s patents and technologies, please visit http://www.tto.hku.hk. Potential industrial/ business partners who are interested to discuss commercialisation opportunities are welcome to contact TTO by email at info@tto.hku.hk or telephone at (852) 2299 0111.
Please visit the website at http://www.med.hku.hk/v1/news-and-events/press-releases for press photos.
Professor Jiandong Huang, Professor of School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, HKU, pointed out that delivering “YB1” with therapeutic proteins and drugs to the tumour tissues can result in tumour regression.
The green signal in the upper part of the photo shows the lung metastasis in breast cancer mouse models in control group, while the lower part of the photo shows that there is no lung metastasis when breast cancer mouse models in treatment group were treated with YB1, indicating that cancer metastasis was completely inhibited with YB1.