Media
HKU Mechanical Engineering Scientists hail breakthrough in cell and tissue mechanics, enhancing understanding of organ formation and embryogenesis
20 Dec 2023
Figure 2. Propagation of a plastic contraction wave within the tissue. (A) Evolution of the average junction tension in each cell. During simulations, the central two-round of cells (i.e., 7 cells at the center) are illuminated and thus activated at t=0 . (B, C) Quantitative heatmaps of junction tension (B) and myosin activity (C) during the propagation of the plastic contraction wave shown in (A). The solid black line indicates that such a wave propagates at a speed of ~1 round of cells per 2 minutes. (D) Simulated (red points) and experimentally observed (green belts) locations of the plastic wavefront, where the normalised myosin activity becomes higher than 0.2 . (E-H) Propagation of the plastic contraction wave is affected by different physical parameters, including the critical activation strain (E), contraction amplitude (F), viscoelasticity ((G), unit: N∙s∙m-1 ), and reaction time ((H), unit: s ).
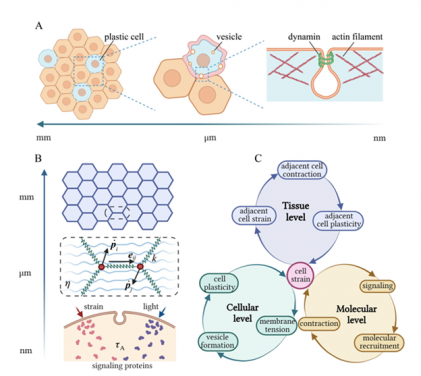
Fig. 1 Cellular plasticity across different scales. (A) Schematics showing that 1) the collective plastic response of cells leads to tissue plasticity at macroscopic scales; 2) formation and scission of membrane vesicles result in irreversible deformation of individual cells; 3) various proteins participate in the initiation and pinching of endocytic vesicles at the subcellular scale. (B) Illustration of the multiscale model where cells are treated as tightly packed hexagons at the tissue level; cell-cell junctions are modelled as springs connecting corresponding vertices at the cellular level; and the activation of signalling molecules (triggered by optical/mechanical stimuli), as well as recruitment of myosin motors to the cell junction, are considered at the subcellular scale. (C) Summary of key processes involved in the development of cellular and tissue plasticity.
Figure 2. Propagation of a plastic contraction wave within the tissue. (A) Evolution of the average junction tension in each cell. During simulations, the central two-round of cells (i.e., 7 cells at the center) are illuminated and thus activated at t=0 . (B, C) Quantitative heatmaps of junction tension (B) and myosin activity (C) during the propagation of the plastic contraction wave shown in (A). The solid black line indicates that such a wave propagates at a speed of ~1 round of cells per 2 minutes. (D) Simulated (red points) and experimentally observed (green belts) locations of the plastic wavefront, where the normalised myosin activity becomes higher than 0.2 . (E-H) Propagation of the plastic contraction wave is affected by different physical parameters, including the critical activation strain (E), contraction amplitude (F), viscoelasticity ((G), unit: N∙s∙m-1 ), and reaction time ((H), unit: s ).
- 1 / 3
- 2 / 3
- 3 / 3
Cells and tissue are known to undergo significant irreversible deformations during processes such as tissue formation and embryo development. A research team led by Professor Yuan LIN from the Department of Mechanical Engineering at the University of Hong Kong (HKU) has made a major new breakthrough in showing how plastic strain develops in individual cells and then propagates within the tissue.
The study, titled "A Mechanism-Based Theory of Cellular and Tissue Plasticity," has been published in the Proceedings of the National Academy of Sciences (PNAS).
“By revealing the biophysical mechanisms behind the development of cellular and tissue plasticity at different scales, the study is a big step in advancing our understanding of processes such as organ formation and embryogenesis,” said Professor Lin. The theoretical framework developed could also provide critical insights for designing innovative strategies in regenerative medicine.
Morphogenesis, the process by which tissues, organs, and organisms acquire complex three-dimensional geometries, involves numerous cells deforming in a highly coordinated and programmed manner. Recent studies have shown that such deformation is often irreversible. But just how cellular and tissue plasticity are developed, as well as the biophysical mechanisms behind them, has hitherto remained a mystery to scientists.
In collaboration with researchers from Nanyang Technological University of Singapore, Professor Lin and his team, including Mr Fuqiang Sun, Dr Chao Fang and Dr Xueying Shao, developed a multi-scale theory, the first of its kind, to show that, in response to optical or mechanical stimuli, the myosin contraction and thermal fluctuation-assisted formation and pinching of endocytic vesicles could lead to the permanent shortening of cell junctions (Fig. 1).
Such plastic constriction can stretch neighbouring cells and trigger their active contraction through mechanochemical feedbacks and their irreversible deformation, ultimately resulting in the propagation of plastic deformation waves within the tissue (Fig. 2).
The theory predicts that endocytic vesicles with a size of around 1-2 µm will most likely be formed. A greater irreversible shortening of cell junctions can be achieved if a long stimulation is split into multiple short ones and the plastic constriction wave propagates at a constant speed within the cell monolayer, all in quantitative agreement with experimental observations.
Professor Lin’s team is among world leaders in the study of cell plasticity and its role in different biological processes. For example, they were the first to show how cellular anisotropy and plasticity facilitate the proper elongation of developing embryos. They have also demonstrated that the capability of tumour cells to undergo plastic deformations is directly correlated with their metastatic potential. This earlier research has been published in prestigious international academic journals such as Science Advances and Physical Review Letters.
Link to the paper: https://www.pnas.org/doi/10.1073/pnas.2305375120
Media enquiries:
Faculty of Engineering
Professor Yuan Lin, Department of Mechanical Engineering (Tel: 3917 7955; E-mail: ylin@hku.hk)
Ms Charis Lai (Tel: 3917 1924; Email: chariskc@hku.hk)

